15:06
News Story
New way for states to cover pricey gene therapies will start with sickle cell disease
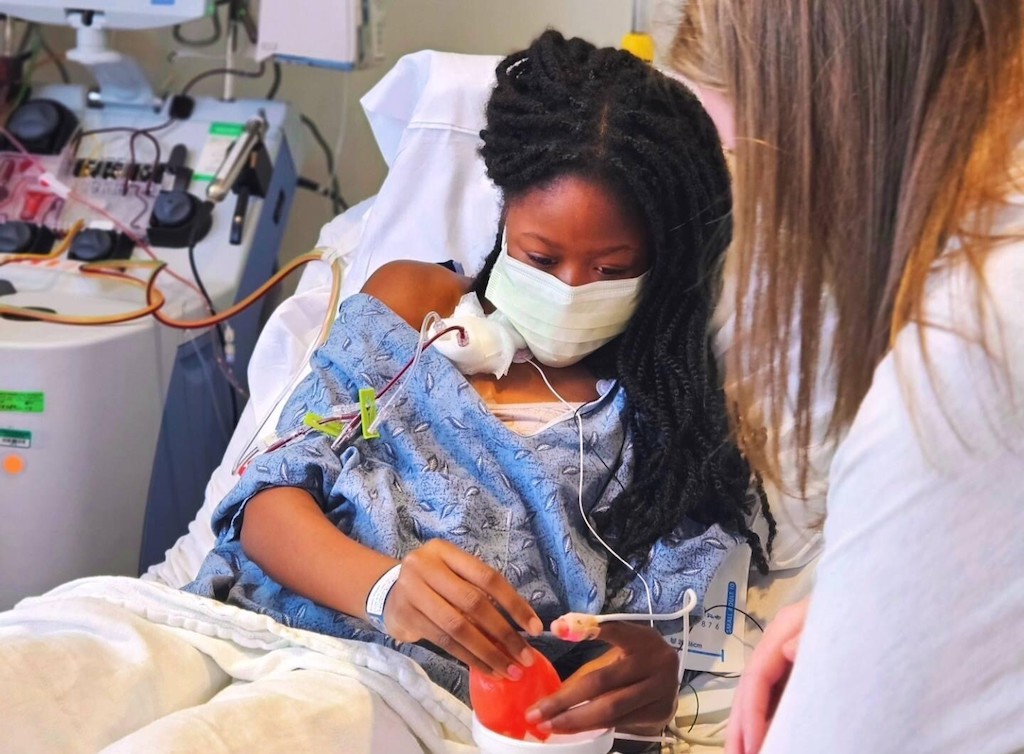
Hajar Tyler, 14, gets a routine blood transfusion at Children’s Healthcare of Atlanta in March. Hajar has sickle cell disease, a rare hereditary blood disorder for which the FDA recently approved two gene therapy treatments. The Centers for Medicare & Medicaid Services has launched a pilot program to help state Medicaid agencies pay for these expensive treatments (Courtesy of Mapillar Dahn, My Three Sicklers Foundation).
The U.S. Food and Drug Administration late last year approved two breakthrough gene therapies for sickle cell disease patients. Now a new federal program seeks to make these life-changing treatments available to patients with low incomes — and it could be a model to help states pay for other expensive therapies.
The new sickle cell treatments have brought hope to those with the debilitating blood disorder, which is hereditary and disproportionately affects Black people. But the therapies come with a price tag of as much as $3 million for a course of treatment, which can take up to a year. Despite those high upfront costs, cell and gene therapies have the potential to reduce health care spending over time by addressing the underlying cause of the disease.
Under the so-called Cell and Gene Therapy Access Model, the federal government will negotiate discounts with sickle cell drug manufacturers Vertex Pharmaceuticals, CRISPR Therapeutics and Bluebird Bio on behalf of state Medicaid agencies, which provide health care coverage to low-income patients. To participate, state Medicaid agencies must agree to prices based on those negotiations, and pledge to provide broad access to the therapies.
The federal government said it will negotiate an “outcomes-based agreement” with the companies, meaning the prices for treatments will be tied to whether the therapy improves health outcomes.
If there are no in-state treatment centers, Medicaid agencies would pay for patients to receive the therapies in another state. Between 50% and 60% of sickle cell patients are on Medicaid, according to federal estimates.
The federal Centers for Medicare & Medicaid Services (CMS) launched the program, which is scheduled to begin next year, in response to President Joe Biden’s 2022 executive order on lowering prescription drug prices.
CMS officials say the framework is being tested with sickle cell disease treatments first, but that other conditions may be added over time.
Several public health organizations, including the American Society of Gene and Cell Therapy and the Alliance for Regenerative Medicine, say the new program could be a model for helping patients with lower incomes afford other gene therapies.
Gene therapies, a rapidly emerging type of treatment, aim to correct genes responsible for rare hereditary diseases. So far, the FDA has approved treatments for a rare inherited eye condition and certain types of cancer, but more therapies are in the pipeline.
Dr. Lakshmanan Krishnamurti, chief of pediatric hematology and oncology at Smilow Cancer Hospital at Yale New Haven Hospital, called the model “path-breaking.”
“This is an initiative the government has taken to responsibly shepherd resources, but at the same time, maintaining access. From a racial equity, health equity perspective, it’s really important that we make this work,” said Krishnamurti, whose sickle cell patients have participated in clinical trials testing gene therapies.
Some states, such as Michigan, are opting to form their own agreements with Bluebird Bio. The company said it’s in talks with Medicaid agencies in 15 other states and is reviewing the CMS framework. Bluebird spokesperson Jess Rowlands said the company looks “forward to working with the agency on an outcomes-based approach that enables access.”
In a statement released this week, Tom Klima, the company’s chief commercial and operating officer, said, “Our commercial approach is built on the principle that people with sickle cell disease insured through Medicaid deserve the same timely access to gene therapy as patients with other forms of insurance.”
In an earnings call last month, Vertex’s chief operating officer Stuart Arbuckle called the new model an “important additional path to access.”
Who is affected?
The majority of sickle cell patients in the U.S. are Black, but people of Hispanic, Middle Eastern and South Asian descent are also disproportionately affected. There are several types of sickle cell disease, but all of them affect hemoglobin, the protein inside red blood cells that carries oxygen. All types of the disease cause the body’s red blood cells to be deformed.
The disease affects an estimated 100,000 Americans. It can cause strokes, severe anemia and episodes of extreme pain, leading to repeated hospitalizations. Those with sickle cell disease have a life span more than two decades shorter than the U.S. average.
The two gene therapy treatments for sickle cell disease recently approved by the FDA, called Casgevy and Lyfgenia, cost $2.2 million and $3.1 million per patient, respectively. The therapies require several other procedures — including chemotherapy prior to the treatments, which involve removing blood cells from a patient and modifying the DNA before re-introducing them in the body.
In Atlanta, Mapillar Dahn’s three daughters have sickle cell disease.
Her oldest daughter, Amatullaah Tyler, who is 20, was hospitalized earlier this month for another pain crisis. At the end of last year, she underwent a hip replacement due to avascular necrosis, which is the death of bone tissue due to lack of blood supply and a complication of the disease.
Sickle cell disease is a leading cause of stroke in children. Dahn’s 18-year-old daughter had her first stroke in second grade, causing neurocognitive and academic issues. She’s had more than 10 surgeries, including a brain surgery. Like her older sister, Dahn’s 14-year-old also suffered learning challenges after a series of ministrokes and is slated for the same brain surgery later this month. Both rely on monthly blood transfusions to prevent future strokes.
Dahn, who founded the nonprofit patient advocacy group MTS Sickle Cell Foundation, also known as “My Three Sicklers,” said she wants Georgia to participate in the model.
Her oldest daughter isn’t currently eligible for the therapy because she is high risk. But Dahn is optimistic for her two younger daughters.
“The hope of it was overshadowed by the access to it,” Dahn said about the new gene therapy treatments. “The bulk of our patient community relies on [Medicaid] for payment. So, I think this is wonderful that they’re testing out this model to not only make it possible for patients to access, but in the long run to somehow make it more affordable.”
Tabatha McGee, executive director of the Sickle Cell Foundation of Georgia, was part of a group that advised federal officials developing the program.
“We’re very excited because it is a step in the right direction,” she said. “This is something we absolutely want to come to fruition. … We’ve never had a singular focus on sickle cell disease to help improve the inefficiencies, to help improve the inequality, inside of the health care system.”
In a statement, the Georgia Department of Community Health told Stateline it is reviewing the framework.
Expanding access
Dr. Lewis Hsu, chief medical officer of the Sickle Cell Disease Association of America, said the gene therapies are an innovative option that don’t come with the same risks as a bone marrow transplant — the only procedure that may be able to “cure” sickle cell disease for some patients — since the treatment uses the body’s own stem cells and doesn’t require a match from another person.
“Having gene therapy available for sickle cell disease is just very exciting,” Hsu said.
Reliable transportation is essential to beginning treatment. In a memo on state obligations, the federal government said states must ensure necessary transportation and travel expenses for both patients and their caregivers.
Hsu, who is also a professor of pediatrics and director of the Pediatric Sickle Cell Center at the University of Illinois Chicago, said transportation and lodging costs are important to consider, as patients come from all over the state or cross state lines to get treatment.
Federal officials told Stateline that multiple states have expressed interest in participating but wouldn’t say which ones.
Illinois’ Medicaid agency told Stateline it intends to participate.
“People with sickle cell disease often confront barriers to accessing treatments that can improve their health outcomes, including the high costs and geographic challenges,” said spokesperson Jamie Munks. “Expanding access to these high-cost treatments can significantly improve the quality of life for people across Illinois who need them, and will contribute to a more equitable health care system overall.”
In Georgia, Democratic state Rep. Gloria Frazier told Stateline she is pushing her state Medicaid agency to enroll. But Frazier noted that because Georgia has not expanded Medicaid under the Affordable Care Act, many patients would miss out.
“I am urging them to definitely participate in the process,” she said. “If we want to really help cure this disease, it has to be affordable.”
Stateline is part of States Newsroom, a nonprofit news network supported by grants and a coalition of donors as a 501c(3) public charity. Stateline maintains editorial independence. Contact Editor Scott S. Greenberger for questions: [email protected]. Follow Stateline on Facebook and Twitter.
Our stories may be republished online or in print under Creative Commons license CC BY-NC-ND 4.0. We ask that you edit only for style or to shorten, provide proper attribution and link to our website. AP and Getty images may not be republished. Please see our republishing guidelines for use of any other photos and graphics.




